Biotech company PTC Therapeutics announced today that their phase III study of Sepiapterin in PKU patients achieved the primary efficacy endpoint.
PTC Therapeutics Announces APHENITY Trial Achieved Primary Endpoint
with Sepiapterin in PKU Patients
PKU (Phenylketonuria) is a rare, inherited metabolic disease, which affects the brain. It is caused by a defect in the gene that helps create the enzyme needed to break down phenylalanine. If left untreated or poorly managed, phenylalanine – an essential amino acid found in all proteins and most foods – can build up to harmful levels in the body. This causes severe and irreversible disabilities, such as permanent intellectual disability, seizures, delayed development, memory loss, and behavioral and emotional problems. There are an estimated 58,000 people with phenylketonuria globally.The pivotal license trial is called APHENITY trial and the randomized withdrawal design was used for the trial even though the randomized withdrawal design was not explicitly mentioned. According to PTC's new release, the APHENITY study is described as the following:
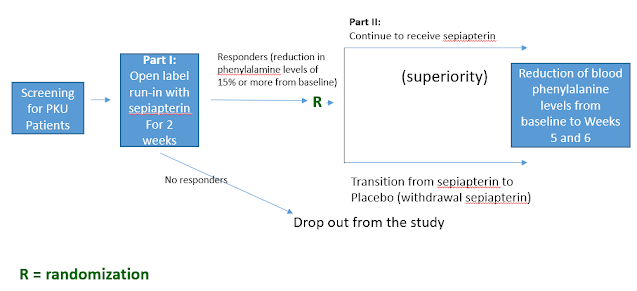
APHENITY was a global double-blind, placebo-controlled, registration-directed study which enrolled 156 children and adults with PKU. Participants were randomized to receive sepiapterin or placebo for six weeks with the primary endpoint being reduction in blood phenylalanine levels. The trial consisted of two parts. Part 1 was a run-in phase, during which all screened subjects received sepiapterin for two weeks. Only those subjects who demonstrated a reduction in phenylalanine levels of 15% or more from baseline in Part 1 were randomized to receive either sepiapterin or placebo in Part 2 of the clinical trial. The primary analysis population consists of those who had greater than 30% reduction in phenylalanine levels from baseline during Part 1 of the trial. The primary outcome measure is the reduction of blood phenylalanine levels from baseline compared to Weeks 5 and 6 in patients from Part 2 of the clinical trial. All patients are eligible to enroll in an open label long term clinical trial designed to further evaluate the long-term safety and durable effect of sepiapterin.
The study design (randomized withdrawal design) can be depicted in the following diagram:
Through the APHENITY trial, it is demonstrated that the randomized withdrawal design can be successfully used in the pivotal study of the rare, inherited metabolic disease.
Refer to the previous posts on randomized withdrawal design:
No comments:
Post a Comment