CQ's web blog on the issues in biostatistics and clinical trials.
Tuesday, September 13, 2016
Free FDA Information Repository
To learn and understand the regulations in drug development and clinical trials, the documents from FDA’s website are extremely helpful. Luckily, there is another website available now for FDA information repository.
Today DIA regulatory affairs community organized a WebEx. Stephen Weitzman, Executive Director from the MedDATA Foundation gave an overview of the IRAI FREE FDA Information Repository. The IRAI repository was initiated under a contract with the FDA with the objective of implementing a new system for cataloging FDA materials in an easily accessible and searchable format for research and training at academic universities and for all other stakeholders interested in FDA’s law, regulation, policies and procedures.
IRAI-Online.Org is open access to all and contains pretty all information from FDA’s website plus additional information that may not be available from FDA’s website or difficult to search on FDA’s website. Some clinical trial related information from NIH website are also included in this new FDA Information Repository website. To request an access, go to IRAI-ONLINE.ORG.
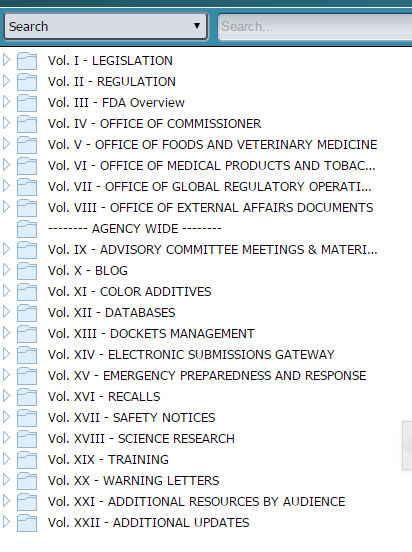
According to the presenter, Stephen Weitzman, the new FDA information repository is cataloged better and more searchable than FDA's website. It is organized by volume I to XXII (volume 1 to 22). The list of volumes is listed below.
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment