“Intent-to-treat (ITT) analyses in superiority trials are nonetheless preferred because they protect against the kinds of bias that might be associated with early departure from the study. In non-inferiority trials, many kinds of problems fatal to a superiority trial, such as non-adherence, misclassification of the primary endpoint, or measurement problems more generally (i.e., “noise”), or many dropouts who must be assessed as part of the treated group, can bias toward no treatment difference (success) and undermine the validity of the trial, creating apparent non-inferiority where it did not really exist. Although an “as-treated” analysis is therefore often suggested as the primary analysis for NI studies, there are also significant concerns with the possibility of informative censoring in an as-treated analysis. It is therefore important to conduct both ITT and as-treated analyses in NI studies. Differences in results using the two analyses will need close examination.”The term “as treated” means that when we do analysis/summaries, the treatment assignment is based on the actual treatment the patients receive, not the treatment the patients are supposed to receive. The concept of “as treated” should be explained in comparison with “as randomized” – a key component for the concept of “Intention-to-Treat” principle.
Typically, ‘Intention-to-Treat’ population can be simply defined as all patients who are randomized. However, the formal definition of the “Intention-to-Treat” population contains several other concepts:
The formal definition of the "Intention to Treat" is usually referred to the one suggested by Fisher, LD et al. in “Intention to treat in clinical trials in Statistical Issues in Drug Research and Development. Edited by Peace KE (1990)”. Intention to Treat population “Includes all randomized patients in the groups to which they were randomly assigned, regardless of their adherence with the entry criteria, regardless of the treatment they actually received, and regardless of subsequent withdrawal from treatment or deviation from the protocol”.
In other words, ITT analysis includes every subject who is randomized according to randomized treatment assignment. It ignores noncompliance, protocol deviations, withdrawal, and anything that happens after randomization. ITT analysis is usually described as “once randomized, always analyzed”. ITT analysis is referred as ‘as randomized’ - the opposite of the term ‘as treated’.
In majority of cases, if all randomized subjects receive their allocated treatments and if there is no randomization error, the ‘as treated’ analysis will be the same as 'as randomized' analysis.
If some randomized subjects do not receive the randomly alloccated treatment and if there are randomization errors, ‘as treated’ population will be different from ITT population or 'as randomized' population.
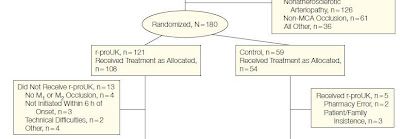
The example below can be used to illustrate the difference between the ‘as treated’ and ‘as randomized’. This is an open label, randomized study to compare the treatment effect of r-ProUK (intra-artery prourokinase + heparin) with control (heparin alone) on acute ischemia stroke - PROACT II Study. Eligible subjects were randomized to r-proUK or Control in 2:1 ratio. 180 subjects were randomized (121 subjects to r-proUK group and 59 to Control group). The problem was that in subjects who were randomized to r-proUK group, 13 subjects never received the treatment and in subjects who were randomized to control group, 5 subjects received the wrong treatment (they were supposed to receive control treatment, but instead received r-proUK).
In this case, to strictly follow the ITT principle, the ITT analysis will include all 121 subjects in r-proUK group and all 59 subjects in Control group - purely based on the randomization, not based on whether or not the randomized subject receive the actual treatment or receive the wrong treatment. The ITT analysis was indeed used in the paper. However, since the PROACT II study was a proof of concept study, the analysis based on 'as treated' population should be used as well.
With 'as treated' analysis, r-proUK group would have 121 subjects and Control group would have 54 subjects (see the comparison and the calculation below).
The
Number of Subjects for Analysis
r-proUK
|
Control
|
|
As Randomized
|
121
|
59
|
As Treated
|
113
(121 randomized subjects - 13 subjects who did not receive
treatment + 5 subjects who were randomized to Control group, but received
r-proUK)
|
54
(59 randomized subjects – 5 subjects who were randomized to
Control, but received r-proUK)
|
Typically, the safety analysis should always be based on the 'as treated' population since it really reflects the safety of patients under each treatment.