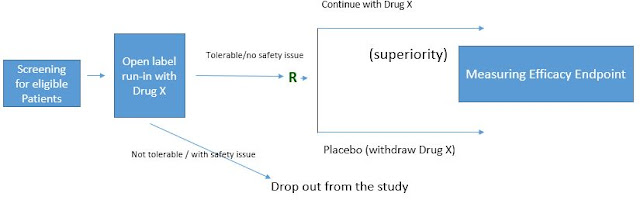
The diagram for a typical randomized withdrawal design will be something like below (with efficacy measure in the run-in period).
or with safety or tolerability as the measure in the run-in period.
The randomized withdrawal design contains a run-in period and a randomized, controlled period. For each period, the criteria will need to be defined. For open-label run-in period, the criteria are needed to define what is considered as 'responder' (for efficacy measure) or 'tolerable' (for safety measure). For the randomized, controlled period where the formal hypothesis testing is based on, the study endpoint will need to be defined.
The table below listed some examples of clinical trials using the randomized withdrawal design. The criteria for the run-in period and for randomized withdrawal period are listed.
Criteria for Responder or tolerability
|
Endpoint for Randomized Withdrawal Period
|
|
10-point INCAT score improve by 1 point
|
Time to relapse where the relapse was defined as 10-point INCAT score worsen by 1 point
|
|
based on symptom and BP response where the response is determined by
improvement of at least one point on a symptom question (Orthostatic Hypotension
Symptom Assessment (OHSA) Item 1) and an improvement in SBP of at least 10
mmHg at 3 minutes post-standing]
|
the mean change from Randomization (Visit 4) to the End of Study Visit (Visit 5) in the OHSA Item 1 (dizziness, lightheadedness, feeling faint or feeling like you might black out) score
|
|
Stable
|
recurrence of severe vasospastic angina leading to study withdrawal
|
|
Median Attacks every 2 weeks
|
Median Attacks every 2 weeks
|
|
|
|
Completed a minimum of 4 weeks of double-blind treatment, reached Visit 4 and completed the 1-week post-treatment washout in the antecedent study (SPD489-325), without experiencing any clinically significant AEs that would preclude exposure to LDX
|
Treatment failure defined as 50% increase (worsening) in Attention Deficit Hyperactivity Disorder Rating Scale (ADHD-RS-IV) total score and greater than and equal to 2 point increase (worsening) in the Clinical Global Impression-Severity of Illness (CGI-S) score observed at any visit during the randomized withdrawal period compared to the respective scores at baseline of randomized withdrawal period.
|
To achieve and maintain clinical stability for at least 12 weeks during the open-label stabilization phase and to have remained on a stable dose of lurasidone for four weeks prior to randomization. Clinical stability was defined as a PANSS total score less than or equal to 70, with PANSS item scores less than or equal to 4 on all positive subscale items and the item for "uncooperativeness”, (item G8), and a CGI-S score less than 4. |
Time to relapse (based on
Kaplan–Meier survival analysis), with relapse defined as greater than and equal to 1 of
the following during the double-blind phase:
(1) An increase of greater than and equal to 25% from double-blind baseline in
PANSS total score and CGI-S worsening of greater than and equal to 1 point for
two consecutive visits no more than ten days apart.
(2) At any single visit, a PANSS item score of greater than and equal to 5 (moderately
severe) on hostility or uncooperativeness, or a
PANSS item score of greater than and equal to 5 on two or more items of unusual
thought content, delusions, conceptual disorganization,
or hallucinatory behavior.
(3) Initiation of supplemental treatment with an antipsychotic
agent other than lurasidone, an increased dose of
an antidepressant or mood stabilizer, an increase in
lorazepam (or benzodiazepine equivalent) dose by greater than and equal to 2
mg/d for at least 3 days, or electroconvulsive therapy.
(4) Insufficient clinical response or exacerbation of underlying
disease reported as an adverse event, as determined
by the study investigator.
(5) Deliberate self-injury or repeated aggressive behavior,
active suicidal or homicidal ideation or attempt.
(6) Psychiatric hospitalization due to worsening
schizophrenia.
Secondary
|
|
A Randomized Withdrawal, Placebo-Controlled Study Evaluating the Efficacy and Tolerability of Tapentadol Extended Release in Patients With Chronic Painful Diabetic Peripheral Neuropathy |
Patients who tolerated tapentadol ER
and had 1-point improvement in average pain intensity from the pre-titration evaluation period to the last 3 days of the open-label titration period were randomly assigned (1:1) to receive tapentadol ER or placebo during a subsequent 12-week double-blind maintenance
phase
|
The primary efficacy endpoint was the mean change in average pain intensity from baseline to week 12
|
Long-term Maintenance of Response Across Multiple Fibromyalgia Symptom Domains in a Randomized Withdrawal Study of Pregabalin |
meeting response criteria for pain
[Z50% reduction in pain 100-mm Visual Analog Scale (VAS) score from OL baseline] and PGIC (self-rating of much
improved or very much improved) at the end of the OL treatment phase
|
Time to LTR (loss of
therapeutic response)
|
Radiologic stable
|
Fraction with radiologic stable disease
|

No comments:
Post a Comment